
Promise Community Health Center and Sioux Center Health are slated to receive initial shipments of COVID-19 vaccines for front-line workers in time for Christmas.
The Health Care Facilities will receive about 400 doses of the vaccine developed by biotechnology company Moderna, pending FDA approval for emergency use authorization, the week of Dec. 20-26.
Front-line workers such as physicians, nurse practitioners, physician assistants and emergency room staff are part of the first tier designated by the state to be eligible for the vaccine. Sioux Center Health is working with Promise Community Health Center in Sioux Center to determine which local health care employees will receive the injection as the first batch will not cover the total employees between the two health organizations.

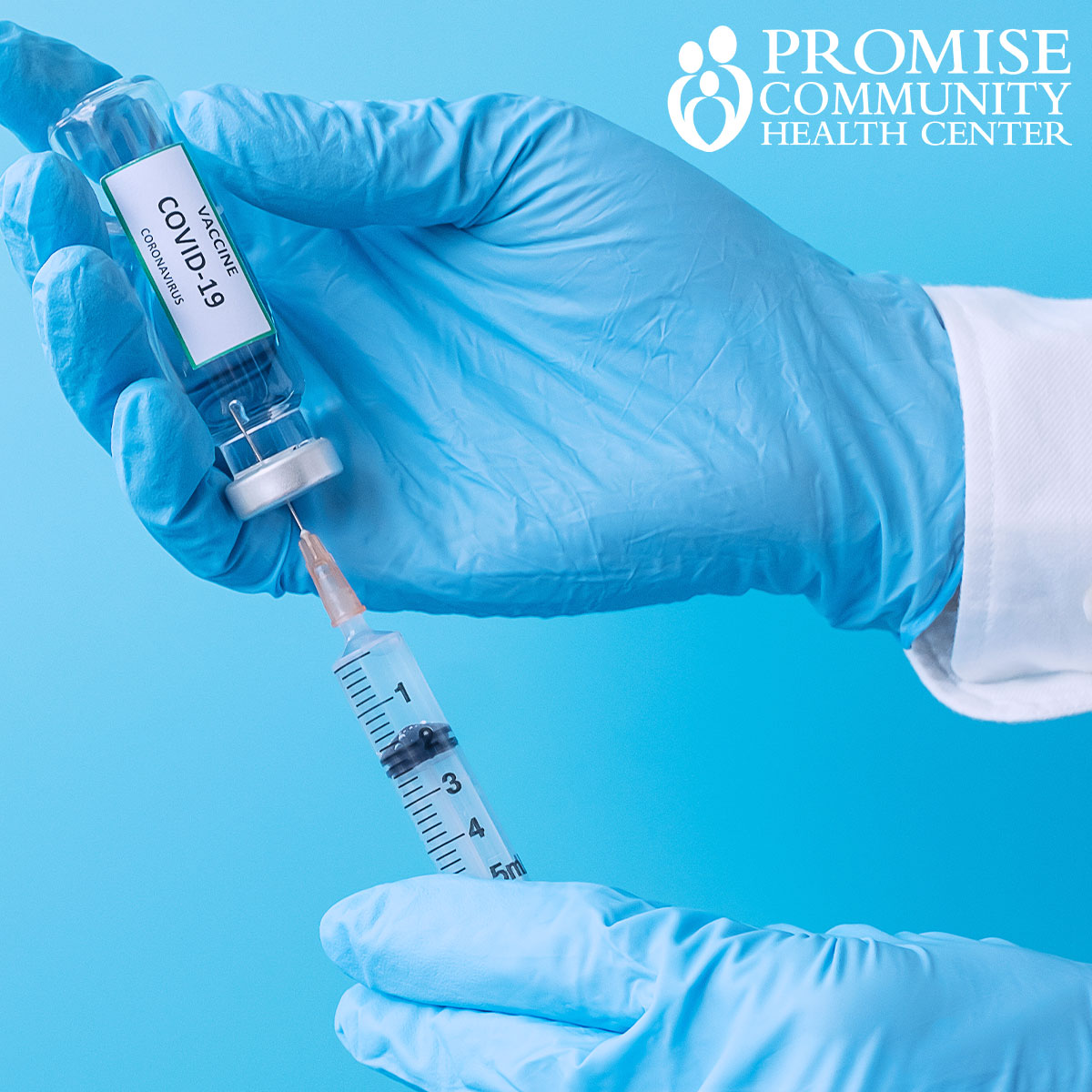
On Friday, Dec. 11, the U.S. Food and Drug Administration approved the first emergency use authorization for a version of the vaccine jointly developed by pharmaceutical company Pfizer and biotechnology company BioNTech for people ages 16 and older.
The Pfizer-BioNTech vaccine will be the version brought to long-term care facilities in Iowa, the vaccination of which will be handled through the Pharmacy Partnership for Long-Term Care Program. Nelson explained that staff from Walgreens pharmacies will set up times for each long-term care facility, such as Royal Meadows in Sioux Center, administer the vaccines to residents and staff.
That’s expected to happen the week of Dec. 28-Jan. 1. However, anyone who is COVID-19 positive is not eligible for the vaccine.
Promise’s clinical pharmacist Dr. Kendra Borchers and Sioux Center Health’s lead pharmacist Dr. Paige Smit encourage the community to consider getting the vaccine once it’s available to the general public.
I know a lot of people are apprehensive or anxious about the vaccine because of the newer technology that they perceive, but the technology they are using has been studied for decades and has been used for other key vaccines like rabies,” Borchers said. “I just want to encourage people that even though it does seem new, it has been studied for quite some time. We’re just using the available tools that we have to develop the vaccine.
Generally the timeline for development of a medication is very linear, Smit said, explaining that a company will start a multi-phase process that begins with the development of a drug, getting approval for it and then starting clinical trials on patients. That’s then followed by the FDA granting approval before manufactures make mass quantities.
“With EUA, the government has essentially said to start building up a supply while the trials are being done and, if it’s approved, then the vaccines are ready more quickly,” Smit said. “It’s safe just as any other medication is approved. I believe this vaccine is trustworthy.”
Promise Community Health Center and Sioux Center Health still encourage people to follow the state’s existing coronavirus restrictions until more vaccinations are made available in 2021.
Read the full Sioux Center News article by clicking on the link here.

